Your cart (0)
Your cart is empty
Tax included and shipping calculated at checkout
Drawer menu
Tax included and shipping calculated at checkout
Have you ever thought about why emeralds are so expensive, or why is there such a difference in price between different types of gems? If so, you are in the right place.
Our planet Earth is made up of rocks, and these rocks are made up of minerals and some minerals are gems or precious stones.
Imagine a recipe. Each recipe uses specific ingredients. And the same goes for minerals - a mineral has a specific chemical composition.
But as with any recipe, there are times when secret ingredients are thrown, and those secret ingredients make everything special.
Contents
Emeralds belong to a great "family"; they belong to the BERYL mineral family. Within this family are other gems such as the aquamarine, heliodor or yellow beryl, morganite or pink beryl, green beryl, red beryl and the Goshenite, also known as a white beryl, which is a colourless beryl.

The aquamarine and the emerald are "sisters", but why do they have such different colours and prices? This is where the secret ingredient comes in:
The "recipe" for a beryl is the following: Be3 Al2 (Si6 O18); that is, it is an aluminium and beryllium cyclosilicate. But if we were to follow the recipe to the letter, if beryl’s contained "ONLY" those ingredients, then the beryls would be colourless, that is, it would be a Goshenite.
Precious stones are "cooked" in the depths of the earth and often during the cooking process small ingredients sneak in and the result is simply amazing.
In the case of the emerald, in the process of the creation of the gem, a very small amount of Chromium or Vanadium was thrown into its composition, resulting in that beautiful green colour.
These little "tiny intruders" that have found their way in to the purity of the chemical compositions of minerals are called: chromophore elements.
Iron is the "intruder" of aquamarine, Heliodor and green beryl; and manganese is responsible for the pink colour of Morganite and red beryl.
How many solar eclipses have you witnessed? I am 47 years old and I have seen very few. Why? Because only very few occur.
The exact same thing happens with emeralds. There are few quality emerald gem, with a good green colour and few internal fractures or fissures.
As you know, emeralds are beryls with small chromium intruders (and sometimes vanadium). There is a common ingredient within the chemical formula of a beryl, known as BERYLLIUM. Well, it is very, very RARE for beryllium and chromium to come together in the same "recipe". This is why the emerald is so rare.

Imagine the earth as though it was a Matryoshka or Russian doll. The smallest doll is the core of the earth, the middle doll the mantle, and the largest is the earth's crust.
Chromium is inside the medium size doll and beryllium within the largest, so the possibility that these two elements can come together is very limited - it is almost a miracle. Something similar happens to our emerald.
We already know that chromium is the "culprit" for the emerald’s green colour. But this is not where everything ends.
When I explained the secret ingredient of the emerald, we saw its chemical formula, and part of this includes another very important element: ALUMINIUM.

For each chromium atom that is introduced to our formula, another of aluminium is removed. The more chrome, the greener and more beautiful our emerald. The problem is that every time it loses an aluminium atom, its structure weakens. This is why the emerald is more fragile than any other beryl mineral.
And if that was not enough, we have to add another difficulty to the creation of the emerald: their genesis or origin.
The earth is formed by rocks, and these can be divided into three large groups: igneous, metamorphic and sedimentary rocks. These rocks are always changing because our planet is alive - this is what is called the rock cycle.
Beneath the earth we find magma, which are molten rocks in a liquid state. These rocks can be cooled and hardened and can come to the surface of the earth thanks to geological processes such as volcanoes. This type of rock is called igneous.
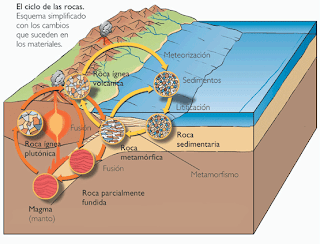
Picture: Rock Cycle. Universidad de Geología de Granada
The rains and the atmospheric phenomena wear away the rocks that are on the surface and these become gravel, sand or pebbles. The wind and rain drag these small bits of rock to the bottom of lakes, rivers and seas.
Little by little, thin layers are formed. As though it were a gigantic lasagne, there are more layers added over time and due to the pressure exerted by both sea water and the layers themselves, they become compact and consolidated, and this is how sedimentary rocks are formed.
In the middle of this great cake that is the Earth, we have an upper section that consists of sedimentary rocks and a lower section made of igneous rocks. And in the middle lies the metamorphic ones.
Metamorphic rocks are formed from igneous and sedimentary rocks, due to high pressures and/or temperatures, as well as magmatic liquids that are introduced into the metamorphic rocks. As a consequence, the stones that grow in this type of rocks tend to have fissures (cracks) or internal fractures.
So, can you guess where emeralds grow? Exactly!
Emeralds grow in metamorphic rocks, where there are more changes in temperature and higher pressure, which is why they tend to have so many fissures and internal fractures.

So, to summarise:
From all this, we can know grasp the idea of why having an emerald in your hand that has good colour, size and few fractures is a miracle of nature. And you pay for miracles.
Thank you very much to Élvira Vilanova (my teacher of gemology at the University of Barcelona) for her help in this blog and in all my learning.